Abstract
Background: Patients(pts) with relapsed or refractory (R/R) AML experience poor response rates and short durations of response. FLAG-IDA salvage therapy has previously demonstrated a composite remission rate (CRc) of 21% with median OS (mOS) of 3.5 months (Roboz, JCO 2014). Combinations of BCL2 inhibition have proven to be synergistic with low intensity and intensive chemotherapy, with improved CRc and mOS rates. Venetoclax with FLAG-IDA demonstrated favorable response rates in the frontline and relapsed or refractory (R/R) setting, with overall response rate (ORR) of 97% and 70% in the Phase IIA (frontline) and Phase IIB (R/R) respectively (JCO 2021). Here we present updated results of the Phase IIb cohort.
Methods: Pts >18 years of age with R/R AML, defined as lack of response after at least 1 cycle of induction or relapsed disease with any prior number of treatments but without prior receipt of venetoclax, with sustained organ function were eligible. Induction consisted of fludarabine (30mg/m2, D2-6), cytarabine (1.5g/m2 D2-6), idarubicin (6mg/m2, D4-5), and Filgrastim(D1-7). Venetoclax was administered D1-14 during induction and D1-7 for consolidation (100mg D1, 200mg D2, 400mg onwards for induction). Consolidation consisted of fludarabine(30mg/m2) and cytarabine (1.5g/m2) for D2-4. The primary objective was evaluation of ORR. Secondary objectives included overall survival (OS), CRc, and duration of response (DOR). Mutation analysis was performed using next-generation sequencing and measurable residual disease (MRD) detection was performed using flow cytometry (sensitivity 10-3-10-4).
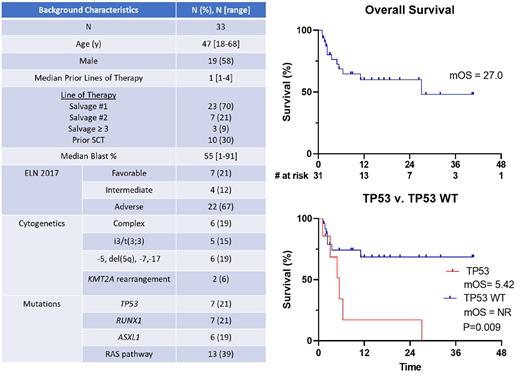
Results: Thirty-three R/R pts have been treated. Baseline characteristics are shown in Table 1. The median age was 47 (range 18 to 68). For 70% of pts, this regimen was Salvage 1. Thirty percent of pts underwent prior SCT. The majority of pts had ELN 2017 adverse risk disease (67%).Complex karyotype and TP53 mutations occurred in 19% and 21% of pts respectively.
Among the 33 pts, 1 patient remains too early for response assessment. The ORR of the remaining 32 pts was 60% (19 pts), with CRc rate of 53% and 12 pts (71% of responders) achieving MRD negativity. ORR and CRc rates in salvage 1 pt were 65% and 61%. By ELN risk, ORR and CRc rates were 100% and 85% in favorable risk, 50%and 50% in intermediate risk, and 48% and 43% in adverse risk. ORR and CRc rates for TP53 wild-type vs TP53 mutant was 68% and 14%, vs 64% and 14%.
The median number of cycles received was 2 (range 1-4) with a median number of cycles to best response of 1 (range 1-2). Among responding pts, 13 (68%) proceeded to allogeneic transplant, including two pts who transitioned to HMA + VEN and sorafenib maintenance, respectively prior to transplant.
At a median follow up of 15.8 months, the OS and DOR were 27 (95% CI 22.3-26.3) months and NR (95% CI 22.4-NR) respectively. Six-month and 12-month survival was 69% and 60%, respectively. Of the 13 pts that proceeding with transplant, median DOR post-transplant has not been reached. OS in TP53 wild-type vs TP53 mutant was NR and 5.4 months with a 12-month OS of 68% and 17% respectively.
Thirty-day and 60 day- mortality was 0% and 12%. Two deaths occurred within 60 days in pts with persistent leukemia (secondary to hemoptysis from known fungal pneumonia and disease).
Conclusion: In a cohort of pts with R/R AML enriched for fit pts with adverse-risk disease, this intensive combination resulted in an ORR of 60% with a CRc of 53%. Seventy-one percent of CRc pts attained an MRD negative remission and 68% of responding pts proceeded to transplant. In conclusion, this regimen is associated with deep remissions and a high rate of transition to transplant inpatients with R/R AML, particularly in TP53 wild-type.
Disclosures
Konopleva:Forty-Seven; F. Hoffman LaRoche: Honoraria; Reata Pharmaceuticals, Novartis and Eli Lilly: Patents & Royalties; Stocks, Reata Pharmaceuticals: Current equity holder in publicly-traded company; AbbVie, Genentech, F. Hoffman La-Roche, Stemline Therapeutics, Amgen, Forty-Seven, Kisoji; Janssen: Consultancy; AbbVie, Genentech, F. Hoffman La-Roche, Eli Lilly, Cellectis, Calithera, Ablynx, Stemline Therapeutics, Agios, Ascentage, Astra Zeneca; Rafael Pharmaceutical; Sanofi, Forty-Seven: Research Funding; Stemline Therapeutics, F. Hoffman La-Roche; Janssen: Membership on an entity's Board of Directors or advisory committees. Takahashi:Symbio Pharmaceuticals: Consultancy; GSK: Consultancy; Novartis: Consultancy; Celgene/BMS: Consultancy; Agios: Consultancy; Mission Bio: Honoraria; Ostuka Pharmaceuticals: Honoraria; Illumina: Honoraria. Loghavi:Astellas: Research Funding; PeerView: Honoraria; GLG: Consultancy; Abbvie: Consultancy, Current equity holder in publicly-traded company; Amgen: Research Funding; QualWorld: Consultancy. Kadia:JAZZ: Consultancy, Research Funding; Iterion: Research Funding; Servier: Consultancy; cellenkos: Research Funding; Ascentage: Research Funding; Genfleet: Research Funding; Astellas: Research Funding; Novartis: Consultancy; BMS: Consultancy, Research Funding; PinotBio: Consultancy; cyclacel: Research Funding; Delta-Fly: Research Funding; Pfizer: Research Funding; Amgen: Research Funding; AstraZeneca: Research Funding; Genentech: Consultancy, Research Funding; Glycomimetics: Research Funding; Astex: Honoraria; Regeneron: Research Funding; Agios: Consultancy; Abbvie: Consultancy, Research Funding. Daver:Kartos and Jazz Pharmaceuticals: Other: Data monitoring committee member; Agios, Celgene, SOBI and STAR Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; Karyopham Therapeutics and Newave Pharmaceutical: Research Funding; Astellas, AbbVie, Genentech, Daiichi-Sankyo, Novartis, Jazz, Amgen, Servier, Karyopharm, Trovagene, Trillium, Syndax, Gilead, Pfizer, Bristol Myers Squibb, Kite, Actinium, Arog, Immunogen, Arcellx, and Shattuck: Consultancy, Other: Advisory Role; Astellas, AbbVie, Genentech, Daiichi-Sankyo, Gilead, Immunogen, Pfizer, Bristol Myers Squibb, Trovagene, Servier, Novimmune, Incyte, Hanmi, Fate, Amgen, Kite, Novartis, Astex, KAHR, Shattuck, Sobi, Glycomimetics, Trillium: Research Funding. Short:Stemline Therapeutics: Research Funding; AstraZeneca: Consultancy; Astellas: Research Funding; Takeda Oncology: Consultancy, Research Funding; Amgen: Consultancy, Honoraria; Novartis: Consultancy; Pfizer: Consultancy. Sasaki:Daiichi-Sankyo: Membership on an entity's Board of Directors or advisory committees; Otsuka Pharmaceuticals: Honoraria; Pfizer: Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding. Borthakur:Pacylex, Novartis, Cytomx, Bio Ascend: Membership on an entity's Board of Directors or advisory committees; Catamaran Bio, Abbvie, PPD Development, Protagonist Therapeutics, Janssen: Consultancy; Astex Pharmaceuticals, Ryvu, PTC Therapeutics: Research Funding. Issa:Novartis, Kura Oncology, Nuprobe: Consultancy; Celgene, Kura Oncology, Syndax, Merck, Cullinan and Novartis: Research Funding. Alvarado:Sun Pharma: Research Funding; FibroGen: Research Funding; Jazz Pharmaceuticals: Research Funding; Daiichi-Sankyo/Lilly: Research Funding; Astex Pharmaceuticals: Research Funding; BerGenBio: Research Funding. Yilmaz:Pfizer: Research Funding; Daiichi-Sankyo: Research Funding. Andreeff:NCI: Membership on an entity's Board of Directors or advisory committees; Glycomimetics: Consultancy; AstraZeneca: Research Funding; German Research Council: Membership on an entity's Board of Directors or advisory committees; CLL Foundation: Membership on an entity's Board of Directors or advisory committees; Daiichi-Sankyo Inc.: Consultancy, Research Funding; Brooklyn ITX: Research Funding; Breast Cancer Research Foundation: Research Funding; Medicxi: Consultancy; Cancer UK: Membership on an entity's Board of Directors or advisory committees; Leukemia & Lymphoma Society: Membership on an entity's Board of Directors or advisory committees; Oxford Biomedical UK: Research Funding; Pinot Bio: Research Funding; Senti Bio: Consultancy, Research Funding; Syndax: Consultancy, Research Funding; Aptose: Consultancy, Membership on an entity's Board of Directors or advisory committees; Oncolyze: Current holder of stock options in a privately-held company; Reata: Current holder of stock options in a privately-held company; Chimerix: Current holder of stock options in a privately-held company; Kintor Pharmaceutical: Research Funding. Jabbour:Spectrum: Research Funding; Amgen: Other: Advisory Role, Research Funding; AbbVie: Other: Advisory Role, Research Funding; Adaptive Biotechnologies: Other: Advisory Role, Research Funding; Pfizer: Other: Advisory Role, Research Funding; Takeda: Other: Advisory Role, Research Funding; Genentech: Other: Advisory Role, Research Funding; Bristol Myers Squibb: Other: Advisory Role, Research Funding. Garcia-Manero:Gilead Sciences: Research Funding; Curis: Honoraria, Research Funding; Aprea: Honoraria; Genentech: Honoraria, Research Funding; Acceleron Pharma: Consultancy; AbbVie: Honoraria, Research Funding; Novartis: Honoraria, Research Funding; BMS: Consultancy, Honoraria, Research Funding; Astex: Consultancy, Honoraria, Research Funding. Ravandi:Astellas: Consultancy, Honoraria, Research Funding; Syos: Consultancy, Honoraria, Research Funding; Novartis: Consultancy; Xencor: Research Funding; Astex/Taiho: Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Honoraria, Research Funding; AstraZeneca: Consultancy; BMS/Celgene: Consultancy, Honoraria, Research Funding; Amgen: Honoraria, Research Funding; Biomea Fusion, Inc.: Research Funding; Prelude: Research Funding; Abbvie: Consultancy, Honoraria, Research Funding. Kantarjian:Astellas Health: Honoraria, Membership on an entity's Board of Directors or advisory committees; NOVA Research: Honoraria; Amgen: Honoraria, Research Funding; Ascentage: Membership on an entity's Board of Directors or advisory committees, Research Funding; Ipsen Pharmaceuticals: Honoraria, Membership on an entity's Board of Directors or advisory committees; ImmunoGen: Research Funding; AbbVie: Honoraria, Research Funding; Daiichi-Sankyo: Consultancy, Research Funding; KAHR Medical Ltd: Honoraria, Membership on an entity's Board of Directors or advisory committees; Jazz Pharmaceuticals: Research Funding; Novartis: Honoraria, Research Funding; Pfizer: Honoraria, Research Funding; Takeda: Honoraria. DiNardo:Cleave: Research Funding; AbbVie: Consultancy, Research Funding; GenMab: Membership on an entity's Board of Directors or advisory committees; LOXO: Research Funding; Astex: Research Funding; ImmuneOnc: Honoraria, Research Funding; Takeda: Honoraria; Servier: Consultancy, Honoraria, Research Funding; Astellas: Honoraria; Kura: Honoraria, Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Honoraria, Research Funding; Foghorn: Honoraria, Research Funding; Forma: Research Funding; Bluebird Bio: Honoraria; Notable Labs: Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees; GlaxoSmithKline: Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria; Jazz: Honoraria; Gilead: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal